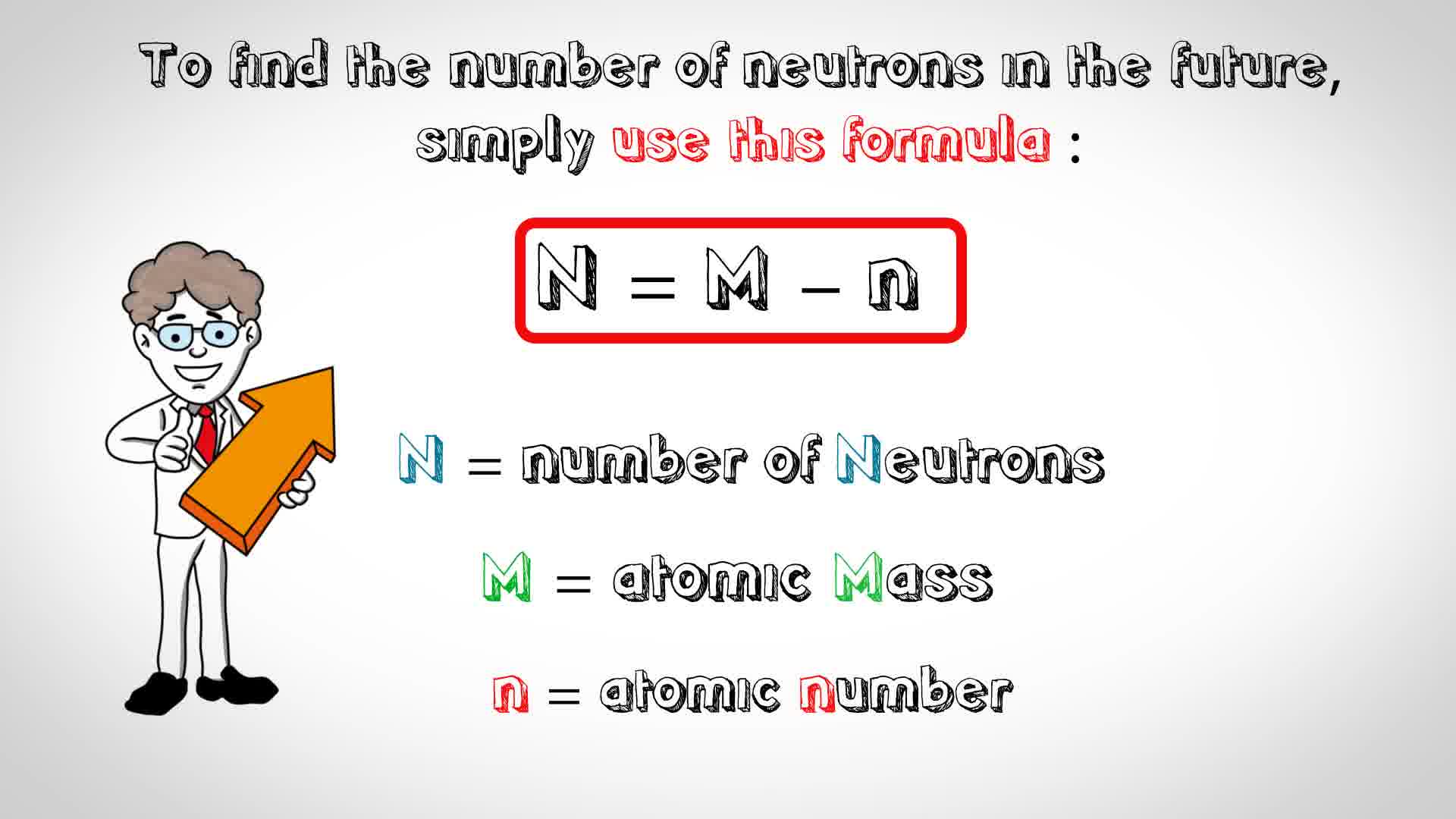Marvelous Info About How To Find Out Neutrons

Molar mass calculator common compounds periodic elements chemical.
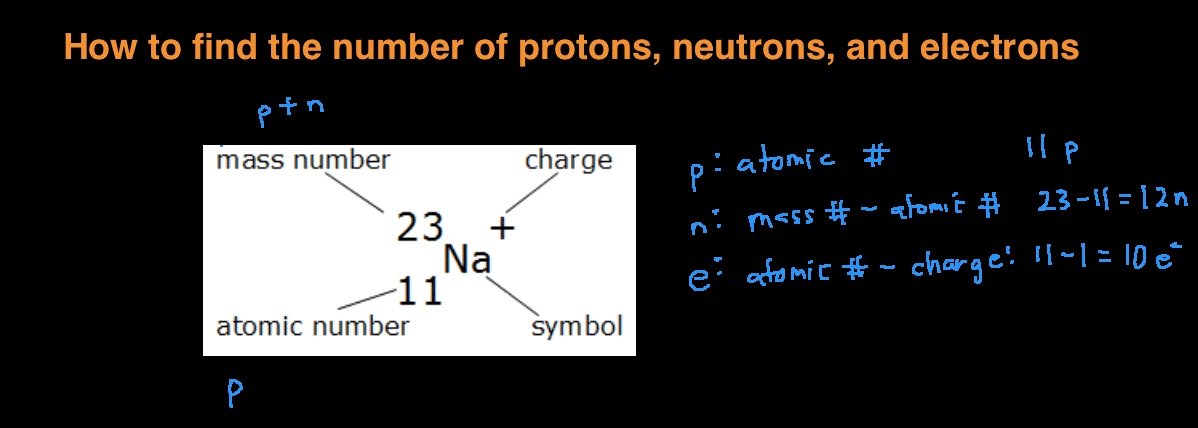
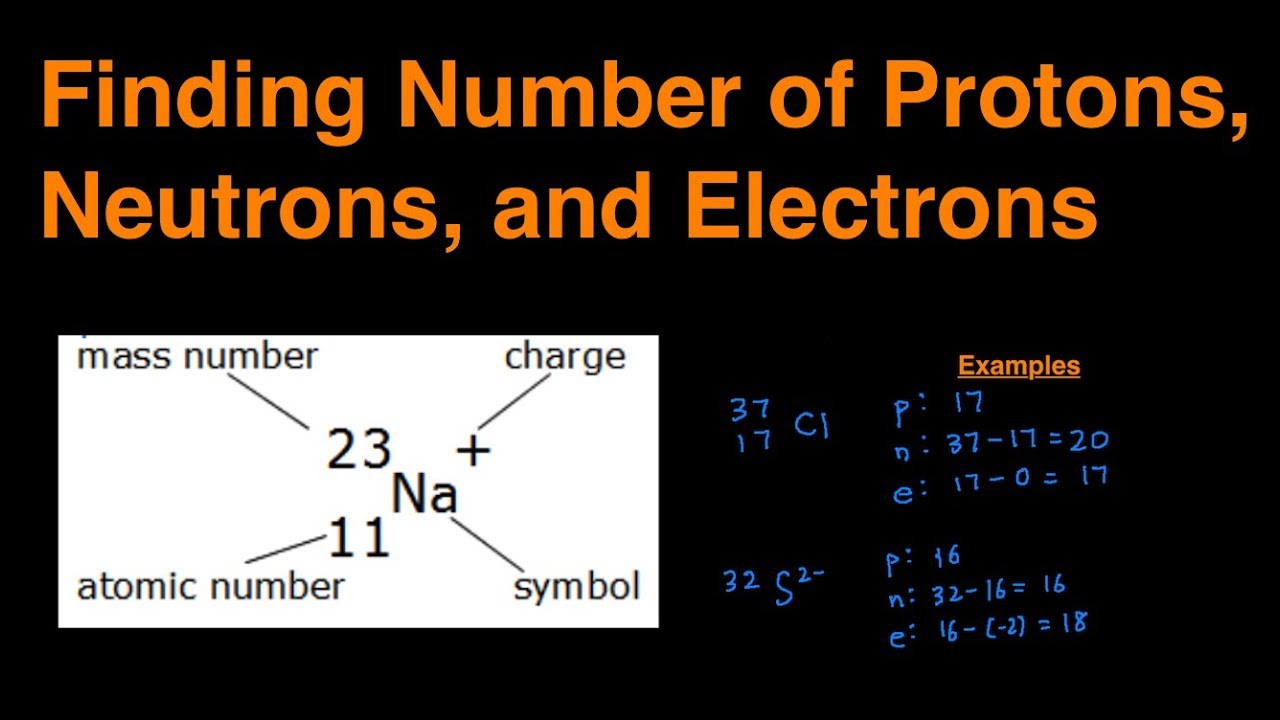
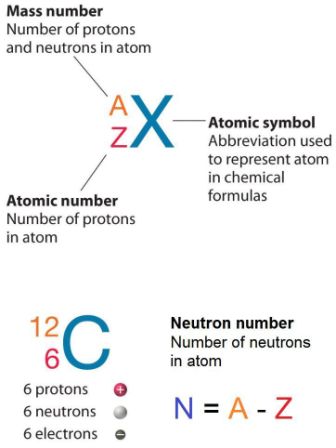
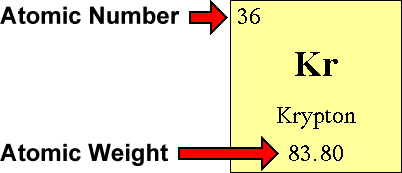
How to find out neutrons. How to find the number of neutrons in an atom: Finding protons neutrons and electrons in an element. Number of protons = atomic number.
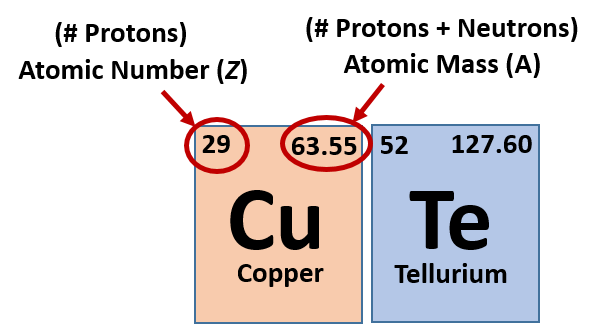
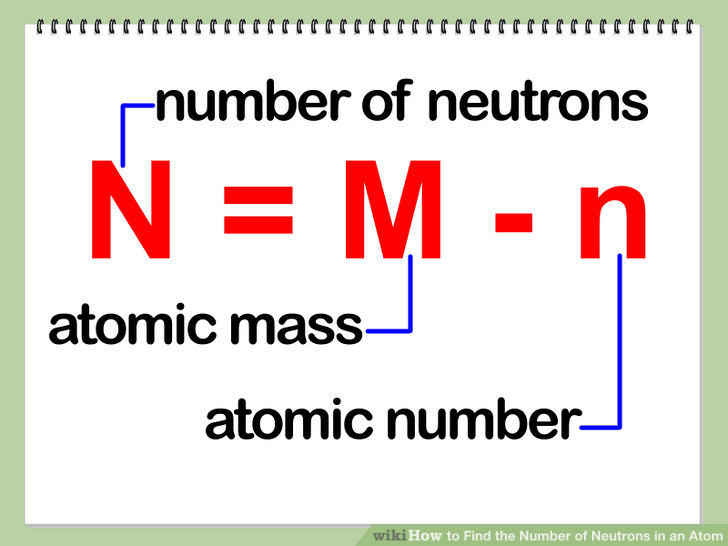
How to calculate the number of neutrons in an atom given the information provided by the periodic table of elements We can determine the number of neutrons by knowing the mass number and atomic number (which is equal to the number of protons) of an element. Number of neutrons = mass.
Finding the number of neutrons the number of neutrons in an atom can be calculated by subtracting the atomic number from the atomic mass. How do you find out how many neutrons a atom has? By using the bohr model of the atom, it is possible to determine the number of protons and.
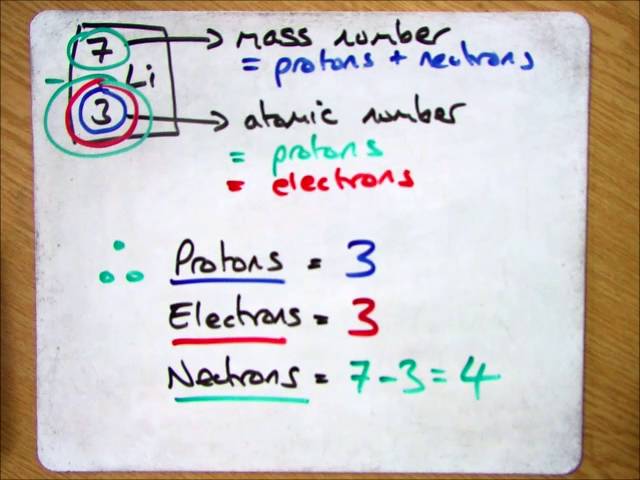
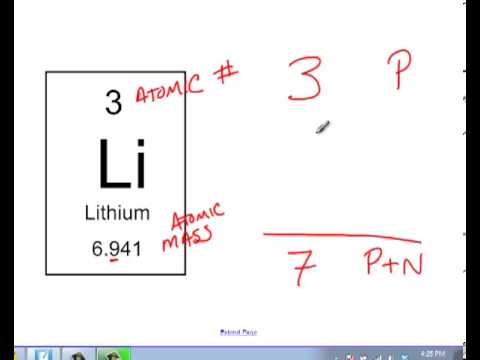
Finding the number of neutrons in an atom is not as difficult as it may seem. The atomic number is the number of protons or electrons. Mass number = atomic number (or number of protons) + number of neutrons
Number of protons = number of electrons = atomic number number of neutrons = mass. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. You find out the neutrons by subtracting the atomic mass from the atomic number.
Number of electrons = number of protons = atomic number. To find the number of neutrons in the atom, subtract the atomic number from the atomic mass. (relative atomic mass) minus (atomic number) explanation:
Thus, the number of neutrons in an element is obtained from the difference between the number of atomic masses and the number of atoms. 11 steps to find the number of neutrons in the future, simply use this formula: That is, neutron number (n) = atomic mass.